There are 4 parts to the particle model theory. They are pretty simple, here we go!
1. All matter is made of tiny particles.
2. The tiny particles are always moving.
3. The particles have spaces between them.
4. Adding heat or energy will make the tiny particles move faster.
Particles behave differently when exposed to heat. The matter becomes more energetic and can go from a frozen state all the way to the hottest plasma if more and more heat (energy) is added to the system.
I have a question! How does snow disappear when the temperature outside is only about 25 degrees Fahrenheit? If you need heat to make molecules vibrate, wouldn't cold make the molecules stay frozen?
States of Matter....kind of like the "twilight zone"....

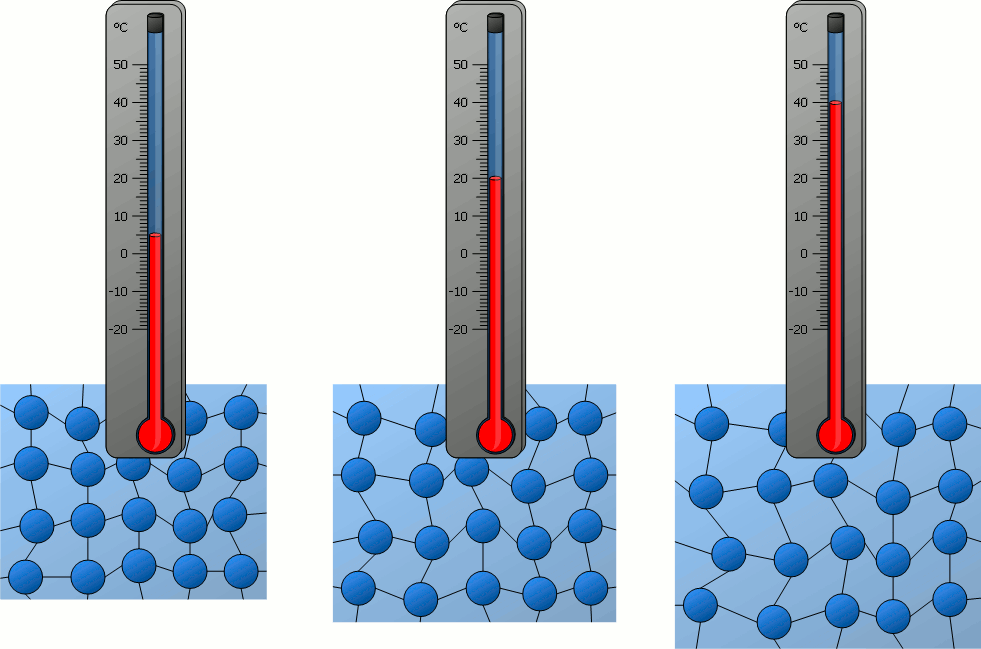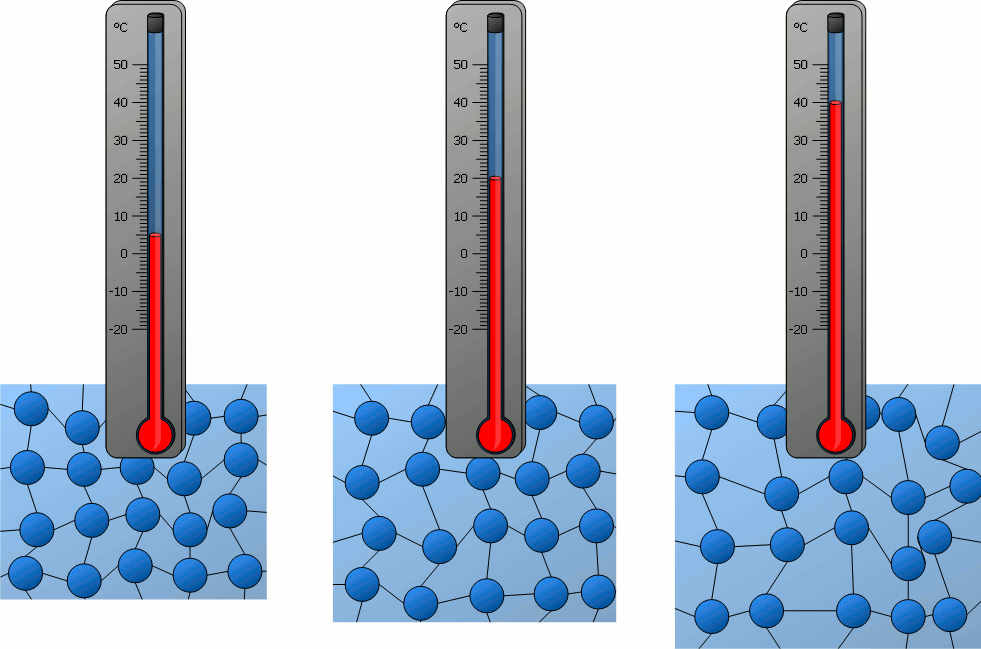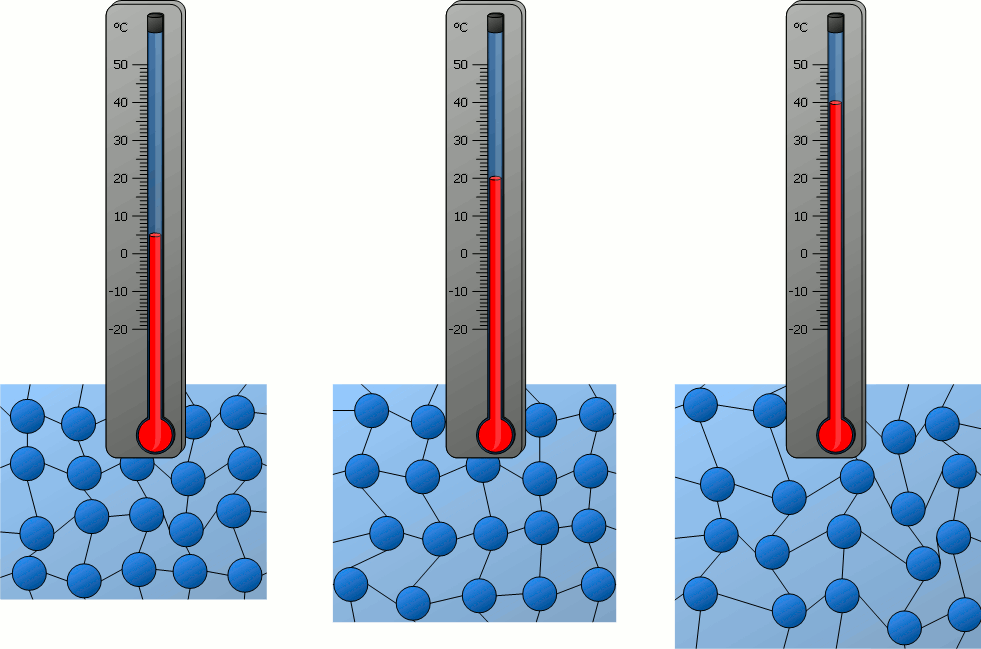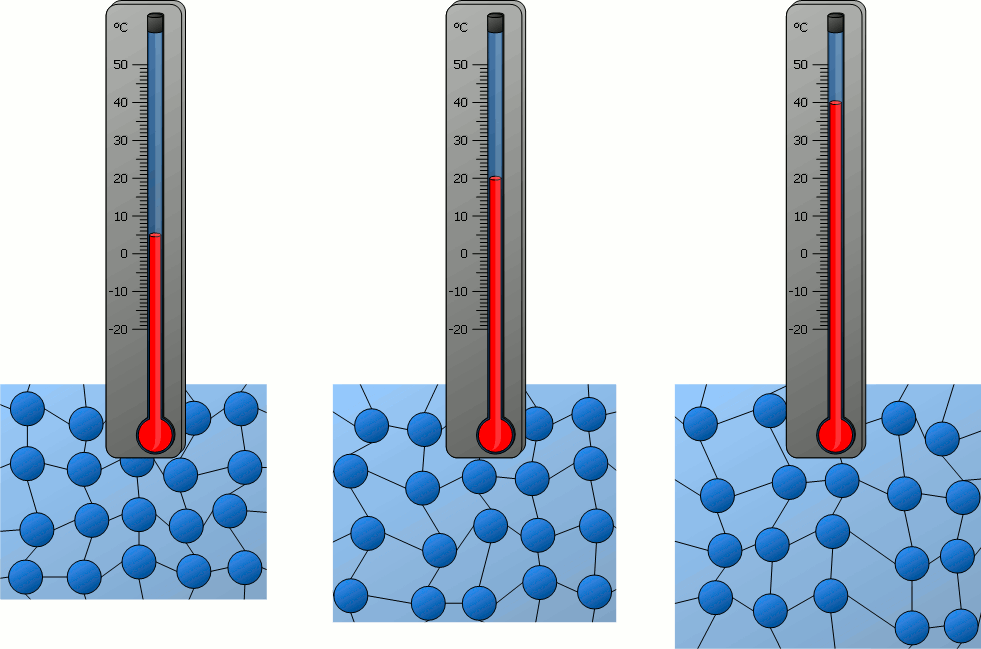



Plasma is really cool. I would stare at it for hours.
ReplyDeleteBefore this, I only ever heard of plasma once
ReplyDelete